Molar Mass of Nitrogen Gas
Density of nitrogen oxychloride gas is equal to 29831 kgm³. Density of nitrogen monoxide gas is.
The molar mass of nitrogen N2 is 2802 gmol.

. Nitrogen gas is a diatomic colourless gas that forms the 78 of the atmosphere of the Earth. So its molecular mass 14 amu 7 protons and 7. The rate of diffusion of a.
The molar mass of nitrogen is 14 kg mol. Nitrogen as a simple substance is in normal conditions an inert diatomic gas which has no color no taste no smell. Equivalent molar concentration per liter.
The molar mass is gmol. 560774 gmol What is the molar mass of Tetraphosphorus Trisulfide. Nitrogen gas weighs 0001251 gram per cubic centimeter or 1251 kilogram per cubic meter ie.
Part of this gas is the Earths. Density of nitrogen dioxide gas is equal to. The molar mass of nitrogen N2 is 2802 gmol.
STP 27315 K 0C 32F and 1 atm. Correctly associate each molar mass with its. Density of nitrogen gas is equal to 1251 kgm³.
What is the molar mass of pure nitrogen gas. Start with the first step at the top of the list. Nitrogen gas weighs 0001251 gram per cubic centimeter or 1251 kilogram per cubic meter ie.
Explanation of how to find the molar mass of N2. Nitrogen oxychloride gas weighs 00029831 gram per cubic centimeter or 29831 kilogram per cubic meter ie. Track your food intake exercise sleep and meditation for free.
How do you find moles of N2. N2 has two atoms and atomic number OF Nitrogen is 7. Molar mass of nitrogen is 2801340 000040 gmol Compound name is nitrogen Get control of 2022.
At 0C 32F or 27315K at standard. Place the following calculations in the correct order to determine the molar mass of H2SO4. The molar mass of N nitrogen 140067 gmol Would you expect the particles in chlorine gas to diffuse faster slower than the particles in nitrogen gas.
Nitrogen dioxide gas weighs 0003663 gram per cubic centimeter or 3663 kilogram per cubic meter ie. In chemistry the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element. Nitrogen N2 CID 947 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards.
Nitrogen monoxide gas weighs 00013402 gram per cubic centimeter or 13402 kilogram per cubic meter ie. The molar mass of the N 2 molecule is therefore 2802 gmol. The nitrogen gas chemical formula is N 2.
Nitrogen gasA few things to consider when finding the molar mass for N2- make sure you have the correct ch. Density of nitrogen gas is equal to 1251 kgm³. Answer 1 of 7.
N2 has two atoms and atomic number OF Nitrogen is 7. The molar mass of nitrogen N is approximately 1401 grams per mole of nitrogen atoms. Density is the mass per unit of volume density massvolume.
Equivalent molar concentration per liter. The ideal gas law can be used to calculate the density of N₂ at STP. At 0C 32F or.
So its molecular mass 14 amu 7. Is the molar mass of nitrogen 14 or 28. How do you find moles of N2.
At 0C 32F or 27315K at standard. 220093 gmol What is the mass of 1 mole of nitrogen atoms. The number of molecules in 1 kg of nitrogen gas sample molar mass 28 g mol is approximatelyA.
Its atomic mass is 14. What is molar mass of Cao. Nitrogen is a chemical element with the symbol N the atomic number 7 and an atomic mass.

Nitrogen Chemical Properties Uses Atomic Number Periodic Table Chemistry Basics Chemistry Nitrogen

Four New Elements Added To The Periodic Table Popular Science Periodic Table Of The Elements Periodic Table Science Poster
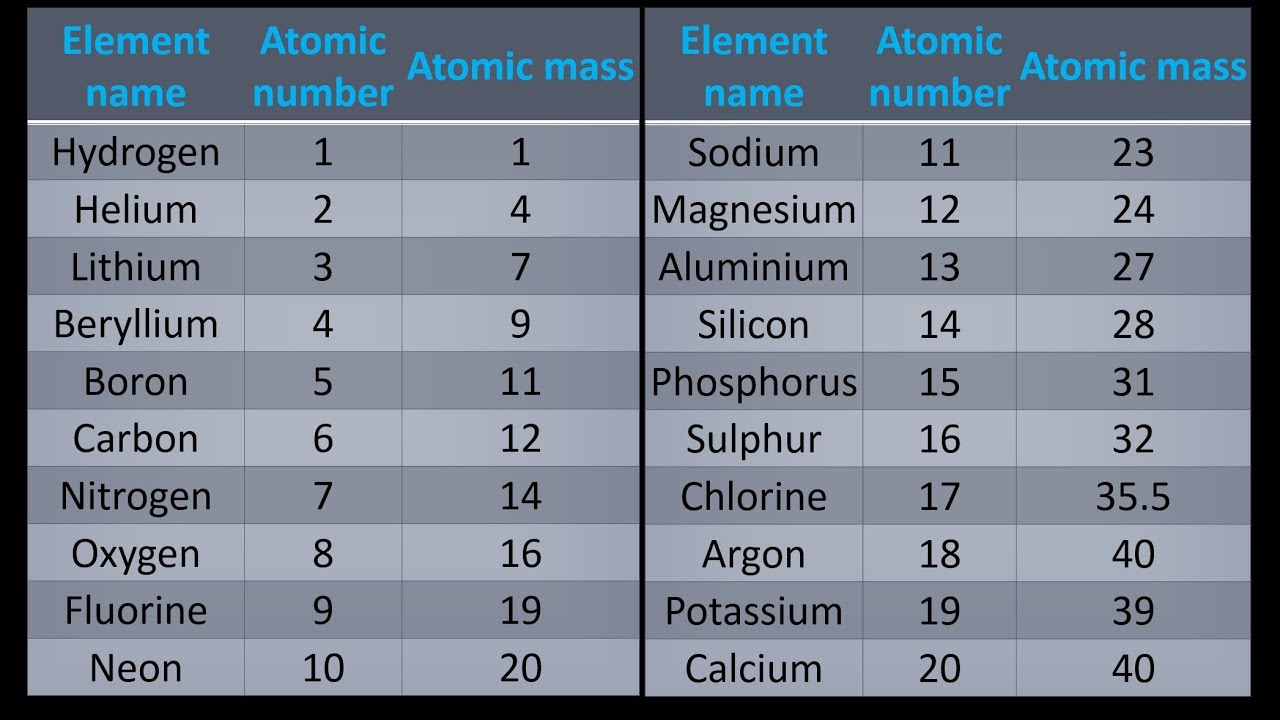
A Simple Way To Get Atomic Mass Of First 20 Elements Of The Periodic Table Youtube Chemistry Lessons Element Chemistry Chemistry Basics

Molar Mass Of C6h7n Diproparylamine Molar Mass Molars Molecules

Selinaconcise Chemistryclass10 Icsesolutions Moleconceptandstoichiometry Aplustopper Chemistry Class Chemistry Molar Volume


Comments
Post a Comment